Physical and chemical changes for class 7 is an important topic for students to consider. Students today actively seek out new information and put it to use. They appear eager to fully comprehend each subject. Students who are looking for chapter notes on physical and chemical changes can find them here.
Physical and chemical changes are essential to the environment. Understanding how to distinguish between chemical and physical changes is a necessary skill for secondary school students. These notes not only assist students in passing regular school exams but also help to crack various Olympiad exams such as CREST Science Olympiad (CSO) for class 7 and similar competitions.
Class 7 Science chapter 6 notes provide a comprehensive explanation of changes in matter, including definitions and additional explanations using appropriate examples.
Notes pdf for chapter 6 of class 7 Science is available to download for free to make it easier for students to revise the concepts later.
Physical & Chemical Changes for Class 7
Every day, we notice various types of changes in our surroundings. The growth of trees, rising of the sun, setting of the sun, different shapes and sizes of the moon, burning of coal, paper, wood, etc. are examples of changes around us.
It is necessary to understand some terms before learning the scientific meaning of changes. i.e. chemical properties, physical properties, irreversible and reversible.
Physical Properties
Shape, size and state of a substance are physical properties. For example; when a rubber band is stretched, its shape changes and this is an example of a change in physical property.
Chemical Properties
Chemical properties are the internal properties of the substance.
For example, curd is obtained from milk but the internal properties of curd and milk are completely different.
Reversible
Things or processes which can be reversed to its original form are called reversible. For example, freezing of water is a reversible change as we can obtain water through the process of melting.
Irreversible
Things or processes which cannot be reversed to its original form are called irreversible. For example, cooking of an egg is an irreversible process.
Differences Between Physical & Chemical Changes
Definition of Physical & Chemical Changes
Physical Change
- Change in which only physical properties of a substance change called Physical Change.
- No new substance is formed.
- Most of the physical changes are reversible.
Chemical Change
- Change in which chemical properties of a substance change called chemical change.
- New substance is formed.
- Most of the chemical changes are irreversible.
- Even in the case of reversible chemical change, the change cannot be reversed by simple physical processes.
In addition to new substance formed, a chemical change may accompany the following:
- Heat, light or any other radiation (e.g. ultraviolet) maybe evolved.
- The sound may be produced.
- There may be change in smell or a new smell may be given off.
- There may be a change in colour.
- A gas may be formed.
Examples for Physical & Chemical Changes
Examples for Physical Change
1. Folding of a paper sheet
A foldation process of a paper is an example of a physical change because we can get the original paper on unfolding it. Additionally, no new substance is formed during this process, which makes it a reversible physical change.
2. Tearing of paper sheet
Each piece is a piece of paper, even after being torn into very small pieces. No new substance is formed during the process so this is a physical change. But as original paper cannot be obtained from the torn pieces of paper, it is an irreversible physical change.
3. Melting of wax
During melting of wax, the state of the wax changes from solid to liquid. From molten wax, solid wax can be obtained. Therefore, this is an example of reversible physical change.
4. Melting of ice into water
During melting of ice, only state of water changes. Water can be changed to ice and vice-versa. Therefore, this is an example of reversible physical change.
5. Freezing of water
Water changes into ice after freezing. In this process; there is only a change happening in the state of water. Though, by melting, water can be obtained back from ice. Hence, it is a reversible physical change.
6. Vaporisation
In the process of vapourization, water changes into vapour. Water can be obtained from vapour, through the process of condensation. Therefore, this is a reversible physical change.
7. Condensation
This is also a reversible and physical change as by the process of vaporisation, water can be changed into vapour again.
8. Stretching of a rubber band
When a rubber band is stretched, only the size of the rubber band changes. Once, the rubber band is released, it returns to its original shape and size. Therefore, this is a reversible physical change.
Examples for Chemical Change
1. Burning of paper, wood, fuel or anything:
When something is burnt, many new substances are formed; especially carbon dioxide is formed in most cases. Once something is burnt, the ash or carbon dioxide cannot be turned into the original substance. Hence, burning of anything is an irreversible chemical change.
Note: Melting of wax and burning of wax are different kinds of change. The burning of wax is a chemical change while the melting of wax is a physical change. Melting of wax is reversible while the burning of wax is irreversible.
2. The reaction between vinegar and baking soda:
Vinegar is an acid (Acetic acid). Chemical name of baking soda is sodium hydrogen carbonate or sodium bicarbonate.
When baking soda is added to vinegar, a hissing sound is produced. This happens because of the production of carbon dioxide. Carbon dioxide turns lime water milky when it passed through it. This happens because of the formation of calcium carbonate. Along with calcium carbonate, water is also formed. The reaction involved in this can be written as follows:
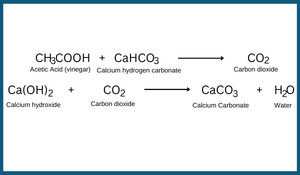
These are examples of chemical changes. In the first case, carbon dioxide is formed as a new substance. In the second case, calcium carbonate is formed as another new substance.
These reactions are irreversible because by a simple physical process an original substance cannot be retrieved.
3. Burning of magnesium ribbon:
Magnesium ribbon on burning forms magnesium oxide and it burns with dazzling light. When magnesium oxide is mixed with water, the ash of magnesium oxide gives magnesium hydroxide. Reaction involved in it can be written as follows:
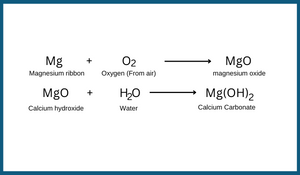
The reaction is an example of irreversible chemical change.
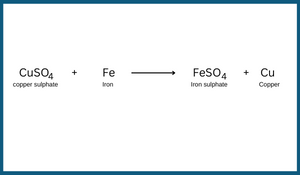
4. Reaction between a solution of iron and copper sulphate:
When an iron nail or shaving blade is dipped in copper sulphate solution then after some time, the colour of solution changes from blue to pale green. This happens because of the formation of iron sulphate. A layer of brown copper also gets deposited over the iron nail or blade. The reaction can be written as follows:
Rusting of Iron
The process of deposition of a reddish-brown layer on teh surface of iron is called rusting. In thai process, a new substance is formed. When articles made of iron come in contact with moisture present in the air, they get rusted. Iron is converted into iron oxide, i.e. rust. The chemical structures of rust and iron are completely different. Rust is iron oxide. Iron is a grey-black material while rust is reddish brown.
Thus, this is an irreversible chemical change. Reaction in rusting can be written as follows:
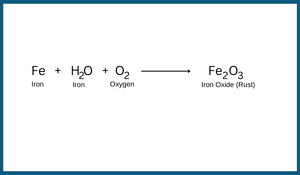
As all the iron slowly turns into rust, the iron article becomes weak. This is the corrosion of iron. Rusting gives a huge monetary loss to the people and nation. For rusting to take place, presence of both oxygen and water should is essential. If either one of these is prevented to come in contact with iron, rusting can be prevented.
Prevention of rusting
- Applying paint to iron objects.
- Applying a layer of grease or oil prevents the iron articles from coming in contact with moist air. This prevents rusting. That is why grease is applied over the chain of bicycles and also over many machine parts.
- By galavanisation: In this process, a layer of non-reactive metal, such as zinc is deposited on iron articles. This layer prevents the iron articles from coming in contact with moisture. Thus, prevents rusting. Water pipes, which are made of iron are galvanized to prevent rusting.
Crystallization
The formation of big and pure crystals of a substance from the saturated solution is called crystallisation. Crystallisation is a physical change. This process is used for purification of some substances.
CREST Science Workbooks
As extra information, more resources are also available for students to practice hard. Students are encouraged to think about using CREST Olympiads workbooks, which are created specifically to assist students in measuring and enhancing their preparation for Olympiad exams.
Although the students have got sufficient clarification about Weather Climate and Adaptation till far but for more detailed reading material, students can purchase CREST Science workbook for class 7 which includes detailed explanations of each topic as well as practice questions with answer key. The book also includes class 7 previous year paper of the CREST Science Olympiad. The book is appropriate for preparing students for CREST Science Olympiad (CSO) and other competitive exams. Also, for regular school studies, this book can also be referred to.
Proceed now to purchase Olympiad books.
Useful Links
CREST Science Olympiad Book (First Chapter) PDF for Class 7 (Free Download)
CREST Olympiads Books for Class 7
Class 7 Notes PDF for Chapter - Physical and Chemical Changes
Interested students preparing for the CREST Science Olympiad (CSO) and many more exams of a similar nature can download the free pdf of notes available for physical and chemical changes for class 7. Also, these notes will undoubtedly help students in preparing for regular school exams as well.
Download Notes PDF for Physical and Chemical Changes
Conclusion
Hopefully, this would have helped students to understand about physical and chemical changes for class 7. We did our best to provide notes for chapter 6 of class 7 Science. Examples of physical and chemical changes are also mentioned to make the concept easier to understand. We would love to continue providing more information on Science topics to students in the future. Don’t forget to download the free pdf.








